Giant Clots: Syncytia via multi-Arginine motif?
Spike exposure melts cells together as Syncytia in lungs and heart - what happens in blood vessels?
This post further discusses my theory of Syncytia-building as one mechanism contributing to Giant Rubbery Clots, driven by the multi-Arginine motif in Spike. Arginine-rich HIV-1 Tat is long known to cause cells to melt together into Syncytia (a driving factor in HIV dementia).
Many of my posts are interested in the multi-Arginine motif that unconventionally materialized in Spike. It has received attention as a Furin Cleavage Site. I have been questioning it as a well-loved Biolab tool derived from HIV Tat, used to transfect foreign genes into the nucleus and to deliver cargo past the Blood-Brain Barrier.
Here’s a strong version of multi-Arginine in the lab:
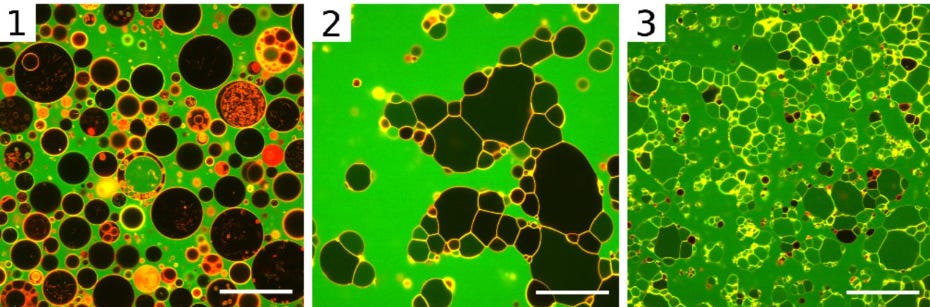
Arginine-rich cell-penetrating peptides induce membrane multilamellarity and subsequently enter via formation of a fusion pore
Allolio C, Magarkar A, Jurkiewicz P, et al. Proc Natl Acad Sci U S A. 2018;115(47):11923-11928. doi:10.1073/pnas.1811520115
“Fluorescence microscopy images showing the effect of R9R9 on GUV with composition 4. From Left to Right: 1, no peptide added; 2, shortly after addition of R9R9; and 3, final state after 1 h. (Scale bars, 50 μμm.)”
“The passive translocation mechanism of arginine-rich cell-penetrating peptides has puzzled the scientific community for more than 20 y. In this study we propose a hitherto unrecognized mechanism of passive cell entry involving fusion of multilamellar structures generated by the cell-penetrating peptides. The geometry of entry for this mechanism is completely different from previously suggested direct translocation mechanisms, leading to another paradigm for designing molecular carriers for drug delivery to the cell.”
Different arginine-rich motifs behave differently and have been developed for different purposes in the lab – how does Spike’s PRRAR motif perform?
Spike exposure has been shown to provoke Syncytia-building in lungs, heart, etc. I have therefore been asking whether Sudden Deaths are due in part to this Arginine-rich motif. Syncytial remodelling in brainstem centers or heart could interfere with vital impulses; Syncytial globs of blood cells combined with Netosis products, fibrin, and damaged endothelial and extracellular matrix components could likewise contribute to the giant rubbery clots. This question should be relatively straightforward to check using the right tests.
To consider my giant clot hypothesis, this post looks at the susceptibility of blood cells to Spike and considers whether each cell type would be theoretically susceptible to Syncytia-building, based on shared characteristics with other cells known to form Spike-driven Syncytia.
-
-
BLOOD: SYNCYTIA CANDIDATES
Are blood cells susceptible to Syncytia-building like other cells are? Considering that Arginine-rich motifs have been honed as a Cell Penetrating Peptide, any cell with a membrane could be a valid target depending on how that motif is positioned. But here we can take a conservative approach and focus on the well-known Spike receptors or if each cell type has demonstrated infection.
Cells present in blood and the vascular endothel lining, roughly:
White blood cells: (1) lymphocytes, (2) monocytes, granulocytes [(3) neutrophils, (4) eosinophils, (5) basophils]; (6) Red blood cells, (7) Platelets, (8) Vascular endothelial cells
There are also hulled fragments such as exosomes, apolipoproteins, and various circulating proteins with clotting or clumping-together relevance. Matrix metalloproteases are a topic in Covid inflammation and endothelial damage, implicating the glycocalyx and extracellular matrix – these are saved for another post.
-
-
(1) Lymphocytes: candidates for Syncytia
DOI: 10.1038/s41392-022-00919-x
-
SARS-CoV-2 productively infects primary human immune system cells in vitro and in COVID-19 patients
Pontelli MC, Castro ÍA, Martins RB, et al. J Mol Cell Biol. 2022;14(4):mjac021. DOI: 10.1093/jmcb/mjac021 (www.biorxiv.org/content/10.1101/2020.07.28.225912v2, this was held in preprint since 2020 - ‘Infection of human lymphomononuclear cells by SARS-CoV-2’
“(SARS-CoV-2) infection is associated with a hyperinflammatory state and lymphocytopenia, a hallmark that appears as both signature and prognosis of disease severity outcome. … We found that monocytes, as well as both B and T lymphocytes, were susceptible to SARS-CoV-2 infection in vitro … In addition, flow cytometry and immunofluorescence analysis revealed that SARS-CoV-2 was frequently detected in monocytes and B lymphocytes from coronavirus disease 2019 (COVID-19) patients.” …
-
(2) Monocytes: candidates for Syncytia
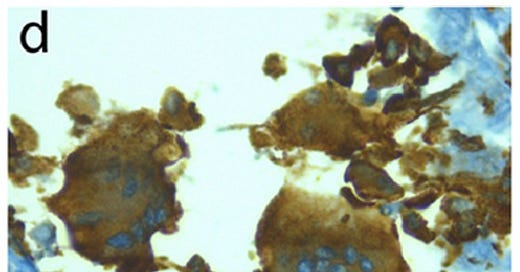
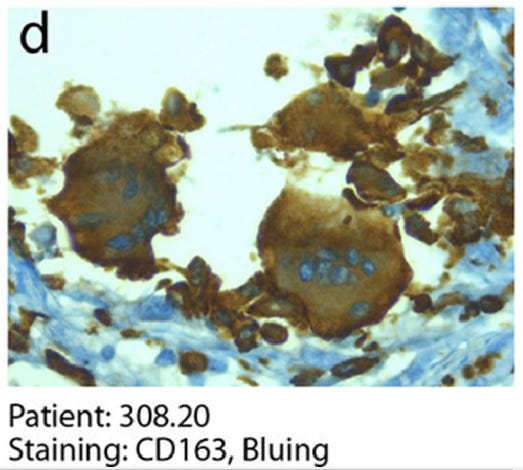
CD163 stains cells of monocyte / macrophage lineage. Shown here are Synctia in a Covid patient.
‘Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology’
Bussani R, Schneider E, Zentilin L, Collesi C, Ali H, Braga L, Volpe MC, Colliva A, Zanconati F, Berlot G, Silvestri F, Zacchigna S, Giacca M. 2020. EBioMedicine 61:103104. 10.1016/j.ebiom.2020.103104.
“The COVID-19 lungs also showed the more occasional presence of CD163-positive syncytia of histiocytic origin.”
-
(3) Neutrophils: candidates for Syncytia
Neutrophils express ACE2, making them theoretically susceptible to Syncytia-building following Spike exposure – compare Ackermann et al. (2021) with Buchrieser et al. (2021) here.
See my other post on Netosis (from Neutrophils) contributing to Covid clots, which is well-established in the scientific literature. Because Neutrophils self-destruct by degranulating, their contribution to Syncytia building may or may not be relevant: this needs to be researched. The exosomes that neutrophils release as part of this process are lipid-hulled and should theoretically be able to merge with lipid-hulled cells - another research opportunity.
Patients with COVID-19: in the dark-NETs of neutrophils.
Ackermann, M., Anders, HJ., Bilyy, R. et al. Cell Death Differ 28, 3125–3139 (2021). https://doi.org/10.1038/s41418-021-00805-z
… “Viable SARS-CoV-2 can directly stimulate human neutrophils to release NETs in a dose-dependent manner (Fig. 4) [53]. SARS-CoV-2-mediated NET-induction requires the angiotensin converting enzyme 2 receptor (ACE2), expressed by neutrophils, the activity of the serine protease TMPRSS2, and virus replication.”
-
Syncytia formation by SARS-CoV-2-infected cells
Buchrieser J, Dufloo J, Hubert M, Monel B, Planas D, Rajah MM, Planchais C, Porrot F, Guivel-Benhassine F, Van der Werf S, Casartelli N, Mouquet H, Bruel T, Schwartz O. EMBO J. 2021 Feb 1;40(3):e107405. doi: 10.15252/embj.2020107405.
“SARS-CoV-2-infected cells … fuse with ACE2-positive neighboring cells. … The TMPRSS2 serine protease … processes both [Spike] and ACE2 and increases syncytia formation by accelerating the fusion process.”
-
(4) Eosinophils: unclear
I haven’t yet found an article describing Eosinophils infected by Spike, but what does seem to be common is patients displaying reduced or undetectable levels of Eosinophils, often correlating with disease severity. A drop in the levels of other blood components sometimes occurs when they get tangled up in clumps. Degranulation is also worth checking.
-
Relationship between blood eosinophil levels and COVID-19 mortality
Yan B, Yang J, Xie Y, Tang X. World Allergy Organ J. 2021;14(3):100521.
… “A progressive decline of eosinophil levels was independently associated with mortality. Moreover, eosinophil levels significantly and positively correlated with platelet and D-dimer levels but significantly and inversely correlated with serum levels of urea, creatinine, aspartate aminotransferase, lactate dehydrogenase, and creatine kinase.
Conclusions: Eosinopenia, if progressively worsening, indicates that COVID-19 patients may progress to critical disease and have a significantly higher chance of mortality. Additionally, eosinopenia correlates with biomarkers of coagulation disorder and those of tissue damage in kidney, liver, and other tissues.”
-
(5) Basophils: unclear
I haven’t found anything intriguing yet on the Basophil topic, and they constitute circa 0.5~1% of White Blood Cells, so they will be overlooked for the moment.
-
(6) Red blood cells: good question
I haven’t found enough information here yet. It is clear that erythrocytes are impacted in various ways – mentioned here e.g. is agglutination – but whether they will be stimulated to melt into other cells as Syncytia is unclear. Here’s some CD147 amusement in the meantime:
-
CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells
Wang K, Chen W, Zhang Z, et al. Signal Transduct Target Ther. 2020;5(1):283. Published 2020 Dec 4. doi:10.1038/s41392-020-00426-x
That’s what the Fourth Mil. Medical University had to say. What do Oxford (Ast.Zen.) and NIH have to say?
doi:10.1128/mSphere.00647-21.
“Basigin, or CD147, has been reported as a coreceptor used by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to invade host cells. Basigin also has a well-established role in Plasmodium falciparum malaria infection of human erythrocytes …. Here, we sought to validate the claim that the receptor binding domain (RBD) of SARS-CoV-2 spike glycoprotein can form a complex with basigin … We show that neither RBD nor full-length spike glycoprotein bind to recombinant human basigin …. Given *the immense interest in SARS-CoV-2 therapeutic targets* …
*“the” or “our” ?
… to improve treatment options for those who become seriously ill with coronavirus disease 2019 (COVID-19), we would caution the inclusion of basigin in this list …. IMPORTANCE Reducing the mortality and morbidity associated with COVID-19 remains a global health priority. Vaccines have proven highly effective at preventing infection and hospitalization, but efforts must continue to improve treatment options for those who still become seriously ill. Critical to these efforts is the identification of host factors that are essential to viral entry and replication. Basigin, or CD147, was previously identified as *a possible therapeutic target* based on the observation that it may act as a coreceptor for SARS-CoV-2, binding to the receptor binding domain of the spike protein. Here, we show that there is no direct interaction between the RBD and basigin, *casting doubt on* its role as a coreceptor and *plausibility as a therapeutic target.”
-
It’s good they noticed this error. Otherwise:
-
A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at Its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody.
Scheim DE. Int J Mol Sci. 2022;23(5):2558. Published 2022 Feb 25. doi:10.3390/ijms23052558
“Rouleaux (stacked clumps) of red blood cells (RBCs) observed in the blood of COVID-19 patients in three studies call attention to the properties of several enveloped virus strains dating back to seminal findings of the 1940s. For COVID-19, key such properties are: (1) SARS-CoV-2 binds to RBCs in vitro and also in the blood of COVID-19 patients; (2) although ACE2 is its target for viral fusion and replication, SARS-CoV-2 initially attaches to sialic acid (SA) terminal moieties on host cell membranes via glycans on its spike protein…
… Many enveloped viral strains, including coronaviruses, initially attach to host cell membranes via glycoconjugate molecules, including those tipped with sialic acid (SA) [25,26,27,28,29,30]. SA is densely distributed on red blood cells (RBCs) as terminal residues of its surface sialoglycoprotein, glycophorin A (GPA), and of its CD147 transmembrane receptors [31,32,33,34]. Through viral bindings to SA surface moieties … SARS-CoV-2 agglutinates RBCs, as established in vitro [35], with such bindings also demonstrated clinically [36].
-
-
Edit: Regarding Plasmodium falciparum, adding a fascinating article that Substack user Castigator pointed out to me:
Antigenic sites in SARS-CoV-2 spike RBD show molecular similarity with pathogenic antigenic determinants and harbors peptides for vaccine development.
Dakal TC. Immunobiology. 2021 Sep;226(5):152091. DOI: 10.1016/j.imbio.2021.152091
"In current study, we performed an integrative approach to predict antigenic sites in SARS-CoV-2 spike receptor binding domain and found nine potential antigenic sites. The predicted antigenic sites were then assessed for possible molecular similarity with other known antigens in different organisms. Out of nine sites, seven sites showed molecular similarity with 54 antigenic determinants found in twelve pathogenic bacterial species (Mycobacterium tuberculosis, Mycobacterium leprae, Bacillus anthracis, Borrelia burgdorferi, Clostridium perfringens, Clostridium tetani, Helicobacter Pylori, Listeria monocytogenes, Staphylococcus aureus, Streptococcus pyogenes, Vibrio cholera and Yersinia pestis), two malarial parasites (Plasmodium falciparum and Plasmodium knowlesi) and influenza virus A."
This list of antigens has relevance to anti-fertility vaccines, which combine a primordial antigen that the body immediately recognizes with motifs that are vital to human reproduction [41 of which were found by Dotan et al. in the Spike (2021, doi: 10.1111/aji.13494)]. See my post ‘Publications worth mentioning'.
-
-
(7) Platelets: candidates for Syncytia
-
SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19
Zhang S, Liu Y, Wang X, et al. J Hematol Oncol. 2020;13(1):120. Published 2020 Sep 4. DOI: 10.1186/s13045-020-00954-7
“Results: We demonstrated that COVID-19 patients present with increased mean platelet volume (MPV) and platelet hyperactivity, which correlated with a decrease in overall platelet count. Detectable SARS-CoV-2 RNA in the blood stream was associated with platelet hyperactivity in critically ill patients. Platelets expressed ACE2, a host cell receptor for SARS-CoV-2, and TMPRSS2, a serine protease for Spike protein priming. SARS-CoV-2 and its Spike protein directly enhanced platelet activation such as platelet aggregation, PAC-1 binding, CD62P expression, α granule secretion, dense granule release, platelet spreading, and clot retraction in vitro… SARS-CoV-2 and its Spike protein directly stimulated platelets to facilitate the release of coagulation factors, the secretion of inflammatory factors, and the formation of leukocyte-platelet aggregates*. …
Conclusions: Our findings uncovered a novel function of SARS-CoV-2 on platelet activation via binding of Spike to ACE2.”
*Leukocyte-platelet aggregates: see Circulating Cell Clusters below. My question: would these aggregate clusters eventually involve Syncytia?
-
(8) Vascular endothelial cells: candidates for Syncytia
CD209 / DC-Sign, otherwise known as HIV gp 120-binding protein:
Withdrawn:
HIV gp 120-binding protein binds Spike:
CD209L/L-SIGN and CD209/DC-SIGN Act as Receptors for SARS-CoV-2
Amraei R, Yin W, Napoleon MA, Suder EL, Berrigan J, Zhao Q, Olejnik J, Chandler KB, Xia C, Feldman J, Hauser BM, Caradonna TM, Schmidt AG, Gummuluru S, Mühlberger E, Chitalia V, Costello CE, Rahimi N. CD209L/L-SIGN and CD209/DC-SIGN Act as Receptors for SARS-CoV-2. ACS Cent Sci. 2021 Jul 28;7(7):1156-1165. doi: 10.1021/acscentsci.0c01537
“Here, we report the identification of CD209L/L-SIGN and the related protein CD209/DC-SIGN as receptors capable of mediating SARS-CoV-2 entry into human cells. Immunofluorescence staining of human tissues revealed prominent expression of CD209L in the lung and kidney epithelia and endothelia. Multiple biochemical assays using a purified recombinant SARS-CoV-2 spike receptor-binding domain (S-RBD) or S1 encompassing both N termal domain and RBD … revealed that CD209L and CD209 interact with S-RBD. … Furthermore, we demonstrate that human endothelial cells are permissive to SARS-CoV-2 infection, and interference with CD209L activity by a knockdown strategy or with soluble CD209L inhibits virus entry. Our observations demonstrate that CD209L and CD209 serve as alternative receptors for SARS-CoV-2 in disease-relevant cell types, including the vascular system. This property is particularly important in tissues where ACE2 has low expression or is absent...”
-
Many cell types: Transferrin Receptor
Adding to the above look at which cells could be theoretically susceptible, here is Transferrin Receptor expression on immune cells. As described in my other posts, Transferrin Receptor was shown to be a target for Spike (Tang et al. 2020, paper held in preprint.) I point out that it is also one of the most widely used biolab tools to deliver cargo past the Blood Brain Barrier. It also happens to be highly expressed on syncytiotrophoblast cells when pregnant, the interface delivering blood and oxygen between mother and child.
-
-
Transferrin receptor is another receptor for SARS-CoV-2 entry
Xiaopeng Tang, Mengli Yang, Zilei Duan, Zhiyi Liao, Lei Liu, Ruomei Cheng, Mingqian Fang, Gan Wang, Hongqi Liu, Jingwen Xu, Peter M Kamau, Zhiye Zhang, Lian Yang, Xudong Zhao, Xiaozhong Peng, Ren Lai. Preprint. doi: https://doi.org/10.1101/2020.10.23.350348).
-
-
Do blood cells form Syncytia?
In the next post, we will look at Spike-triggered Syncytia in various cells and see what information is available on Circulating Cellular Clusters, which have been observed by a number of researchers.
-
Circulating Cellular Clusters Are Correlated with Thrombotic Complications and Clinical Outcomes in COVID-19 [abstract]
Dorken Gallastegi A, Naar L, Van Cott EM, Rosovsky RP, Gregory DJ, Annamalai D, Lee J, Kaafarani HMA, Velmahos G, Tompkins RG, Frydman GH. Res Pract Thromb Haemost. 2021; 5 (Suppl 2). https://abstracts.isth.org/abstract/circulating-cellular-clusters-are-correlated-with-thrombotic-complications-and-clinical-outcomes-in-covid-19/.
… “Blood samples were collected between July – August 2020* from patients with a positive SARS-CoV2 PCR treated at a large academic medical center.
*I would like to see a comparison of blood sample cellular clusters now after several shots.
Imaging flow cytometry was used to detect various circulating cellular clusters, including: platelet (plt)-leukocyte aggregates (PLAs: 1 leukocyte + plt), leukocyte clusters (LC: >2 leukocytes ± any other cell) and platelet-erythrocyte aggregates (PEAs: >1 erythrocyte + plt) (Figure 1). Cluster phenotypes were compared in patients with and without COVID-19 and were retrospectively correlated with clinical outcomes.
… Conclusions: Circulating cellular clusters are correlated with significant clinical outcomes and cluster phenotypes appear to be associated with specific outcomes, including thrombotic events. These immuno-thrombotic complexes may play a significant role in the development of thrombosis and resultant end-organ damage.”
-
-
Edit, Jan 7 2023: Dr. James Lyons-Wyler at Popular Rationalism just put out this post with some familiar concepts:
-
-
Thank you for reading and for your interest in my hypotheses. Feel free to email with questions or comments.
-
-
Articles cited here are only for informational or educational purposes. Please refer to the copyrights of the owners. This post presents original hypotheses, no claims are being made.